
For eligibility, patients had to be naïve to chemotherapy or systemic therapy for recurrent or metastatic squamous cell carcinoma of the oral cavity, the oropharynx, the larynx, or the hypopharynx have an ECOG performance status of ≤1 and have a PD-L1 biomarker analysis for stratification. The data cutoff for the updated analysis was February 25, 2019. Of note, the difference in PFS for this population was not statistically significant (HR, 0.99 P=. “This happened despite the fact that pembrolizumab does not lead to as high a response rate and had a somewhat shorter PFS ,” she said in an interview with Targeted Therapies in Oncology.
The median OS with pembrolizumab was 14.9 months versus 10.7 months in the control arm. The DOR was substantially longer with pembrolizumab, and the safety profile was favorable compared with EXTREME.”Īt the European Society for Medical Oncology 2018 Congress, data presented by Barbara Burtness, MD, co-director of the Developmental Therapeutics Research Program and professor of medicine (medical oncology) at Yale Cancer Center in New Haven, Connecticut, showed that pembrolizumab was superior to the EXTREME regimen for OS in the CPS ≥20 population and that the hazard ratio for death for this patient population was 0.61, which was considered to be highly statistically significant. “With regard to pembrolizumab monotherapy when compared with EXTREME, resulted in superior OS in the PD-L1 CPS ≥20 and ≥1 populations, and it was noninferior…in the total population. The duration of response was longer, and the safety profile was comparable between the 2 regimens,” said Rischin, director of the Division of Cancer Medicine and head of the Department of Medical Oncology at Peter MacCallum Cancer Centre in Melbourne, Australia. “Pembrolizumab when combined with platinum and 5-FU compared with EXTREME resulted in superior overall survival in the PD-L1 CPS ≥20, CPS ≥1, and total populations. In patients with a combined positive score (CPS) ≥20 receiving pembrolizumab monotherapy, investigators observed a 42% reduction in the risk of death versus the control.
KEYNOTE 048 RESULTS PLUS
In the overall population, pembrolizumab and chemotherapy reduced the risk of death by 28% versus cetuximab (Erbitux) plus platinum chemotherapy and 5-FU, known as the EXTREME regimen, in all patients regardless of PD-L1 status.

KEYNOTE 048 RESULTS TRIAL
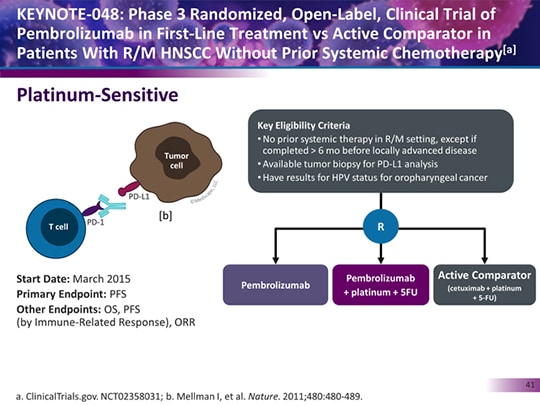
Updated results of the phase III KEYNOTE-048 trial comparing pembrolizumab (Keytruda) as monotherapy and in combination with platinum chemotherapy and fluorouracil (5-FU) with standard-of-care chemotherapy support the use of the PD-1 inhibitor in the frontline for patients with recurrent or metastatic head and neck squamous cell carcinoma, according to Danny Rischin, MD, who presented the results of the final analysis at the 2019 American Society of Clinical Oncology Annual Meeting.


 0 kommentar(er)
0 kommentar(er)
