
Radiation: A Review of Human Carcinogens. Carbon-14 CH3 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards.
#Carbon 14 protons skin
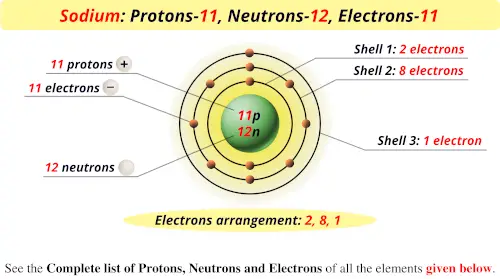
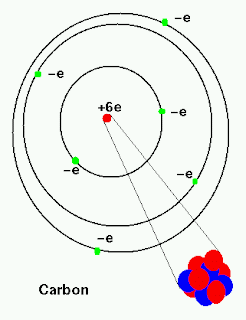
Ingestion, inhalation, puncture, wound, skin contamination (absorption) LPSC XX 291 CARBON-14 PRODUCTION BY 155-Mev PROTONS IN METEORITES E.L. Six electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 6 protons (red) and 8 neutrons (blue). Boro Protones Neutrones Electrones Configuración electrónica. Categorías Elements - Protons and Neutrons Etiquetas Elements - Protons and Neutrons Navegación de entradas. Predicted LC-MS/MS Spectrum - 40V, Negative Diagram showing the nuclear composition and electron configuration of an atom of carbon-14, an unstable isotope of the element carbon (C). El carbono 14 también se puede producir en la atmósfera mediante otras reacciones de neutrones, que incluyen en particular 13 C (n, ) 14 C y 17 O. This means that a neutron in a carbon 14 atom turns into a proton and an. Predicted LC-MS/MS Spectrum - 20V, Negative Carbon 14 decays into nitrogen 14 by beta decay. Thus, the isotope notation for Carbon- 14 is. Carbon has the atomic symbol C, and it has 6 protons. In Carbon- 14, 14 is the mass number (sum of protons and neutrons). Splash10-014i-9000000000-c888af3d1348fef91ee6 where Z is the atomic number (number of protons), A is the mass number (sum of protons and neutrons), and X is the element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The Group 14 elements tend to adopt oxidation states of +4 and, for the heavier elements, +2. Carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Each of these elements has only two electrons in its outermost p orbital: each has the electron configuration ns2np2.
Predicted LC-MS/MS Spectrum - 10V, Negative The carbon family, Group 14 in the p-block, contains carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl).
Predicted LC-MS/MS Spectrum - 40V, Positive Predicted LC-MS/MS Spectrum - 20V, Positive Predicted LC-MS/MS Spectrum - 10V, Positive These are acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms. Belongs to the class of organic compounds known as alkanes.


 0 kommentar(er)
0 kommentar(er)
